 |
CLIA number is required for this purchase.
Note: This product can only ship to a medical or an educational facility.
Rapid detection of SARS-CoV-2 will play a key role in the global spread of the virus.
Affordable and sensitive test that does not require an additional reader, with a processing time of 15-20 minutes.
Features and Benefits
- Detects SARS-CoV-2 nucleocapsid protein antigen
- Rapid results in 15-20 minutes
- Anterior nasal swab specimen collection
- Identifies acute infection with a 92.31% sensitivity and 99.04% specificity
- For use in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance or Certificate of Accreditation.
- The confirmed LoD for the GenBody COVID-19 Ag is 1.11x 10 TCID50/mL
- Selected by NIH for the Rapid Acceleration of Diagnostics program, for the use production of the GenBody COVID-19 Ag test.
- Detects different SARS-CoV-2 variants
Note: - The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for the detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1) unless the declaration is terminated or authorization is revoked sooner
Test Procedure
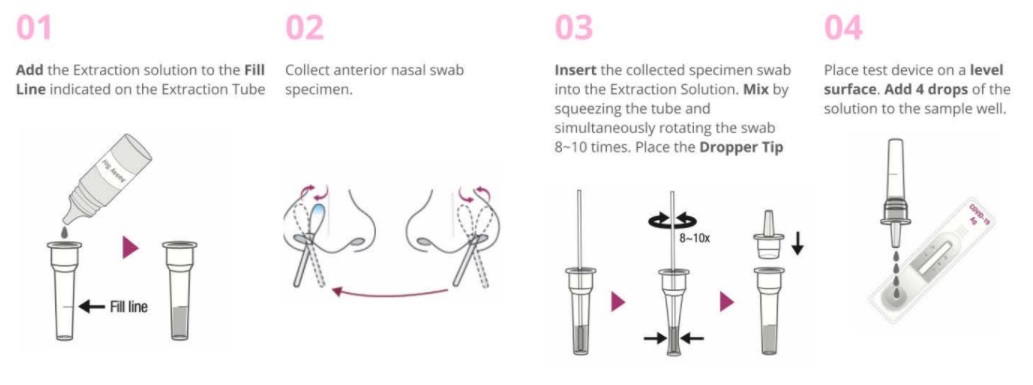
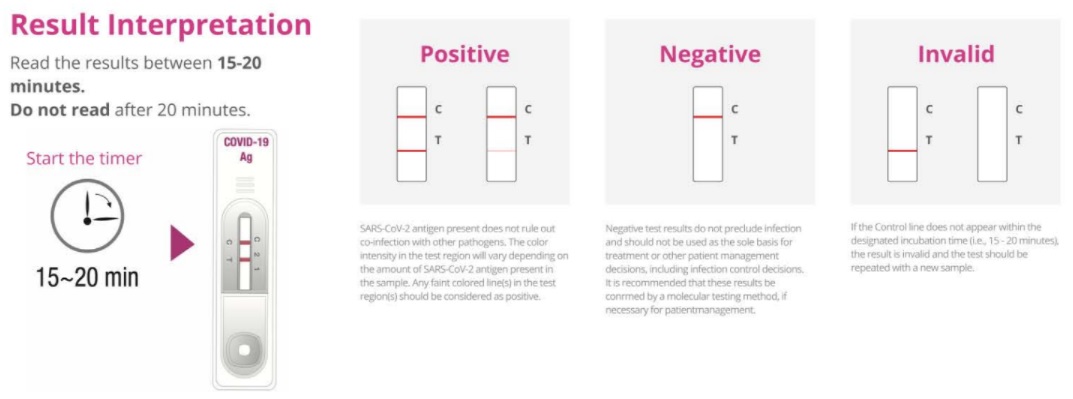
Result Interpretation
Materials Included- 25 Single Use Test Devices Individually Foil-Pouched
- 2 Bottles of Extraction Solution
- 25 Single Use Extraction Tubes
- 25 Single Use Dropper Tips
- 25 Sterilized anterior nasal swabs
Technical Specifications
|
|
 |


 Gen body Covid-19 rapid Antigen test kit (25/box)
Gen body Covid-19 rapid Antigen test kit (25/box) 


